Adding a water-repelling layer to a metal ball means it can sit submerged without boiling explosively, scientists have discovered.
Writing in Nature, Northwestern University researcher Neelesh Patankar and his colleague Ivan Vakarelski, coated 20mm diameter stainless steel balls with a commercially-available material called "Glaco Mirror Coat".
This superhydrophobic layer repels water and is stable to temperatures of over 400°C. Compared with control, untreated balls heated to this temperature and dropped into cold water where they produced a fierce explosive boiling, the superhydrobic balls - in Petankar's own words - "did nothing". In fact, fast-frame video footage of the experiments show that the balls become surrounded with a layer of water vapour.
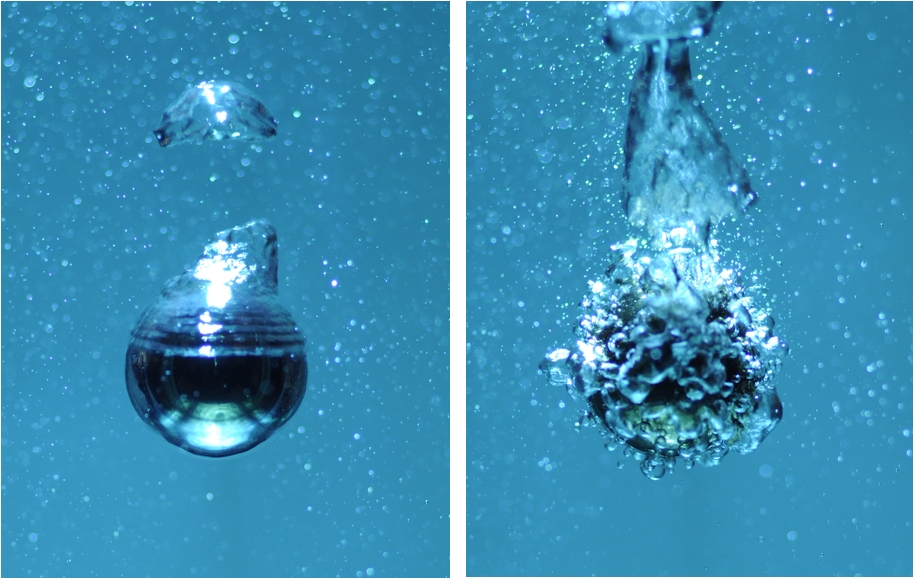 Apart from being a relatively poor heat conductor, this prevents the liquid touching the ball surface, so it cannot boil off.
Apart from being a relatively poor heat conductor, this prevents the liquid touching the ball surface, so it cannot boil off.
The researchers think that the process works because the applied coating consists of nanoscale ridges and grooves. Liquid, they speculate, sits atop the ridges but cannot flow down into the grooves owing to the hydrophobicity of the surface. So water vapour fills the groove instead, keeping the liquid at bay.
Why this is important is that explosive boiling occurring when liquids contact hot surfaces can be problematic in some industries. Being able to prevent this could be a major processing advantage.










Comments
Add a comment